Cleanliness in Medical Engineering
Whether instruments, implants or accessories are involved, cleaning is an imperative step in the manufacturing process for medical devices.
Modern cleaning processes allow compliance with the high degrees of cleanliness required in medical device manufacturing.
Share




Modern cleaning processes allow compliance with the high degrees of cleanliness required in medical device manufacturing. These processes are reliable and reproducible, with short processing times that contribute to enhanced value creation.
Sticky residues from the manufacturing process, such as release agents, machining media, chips and dust, must be removed from medical devices during the cleaning process, but while these steps must be reliable and documentable, they must also meet biocompatibility requirements. The cleaning industry offers various cost effective and ecological processes to fulfill these requirements. Methods include wet chemical cleaning processes, cleaning with carbon dioxide, and plasma processes, along with various combinations of these and other processes.
Wet Chemical Cleaning
The effectiveness of wet chemical cleaning processes such as immersion, spray and ultrasonic cleaning is determined by interaction amongst the dissolving performance of the utilized cleaning agent and the cleaning system’s process technology. The most commonly used cleaning media are aqueous cleaning agents and solvents. Essential criteria for the selection of the ideal cleaning process include the material to be cleaned, type of contamination, part shape, cleanliness requirements with regard to film-like contamination and particulates, as well as production throughput. The most suitable process can be selected on the basis of these factors, and the final choice is frequently corroborated by cleaning tests conducted by equipment or cleaning agent manufacturers.
Aqueous cleaning agents are based on an organic or an inorganic builder and tensides. The latter are able to “push” themselves in between the contamination and the material to be cleaned and dislodge non-polar contamination such as oil and grease, as well as polar contamination (such as emulsions, salts and particles). In order to prevent residues left by the cleaning agent or surface spots that would impair quality or biocompatibility, a multi-stage rinsing process is usually required—often with deionized water in the final rinsing stage(s). Continuous monitoring of the bath and bath replacement at regular intervals are necessary for consistently good results. Solvent systems that are available as chlorinated hydrocarbons (CHC), non-halogenated hydrocarbons, modified alcohols and polar solvents are distinguished by great diversity with regard to the type of material to be cleaned. Grease, oil, particles, and so on can be removed.
Corresponding legal regulations regarding emissions limits and work safety apply to the use of solvents, and these must be taken into consideration in modern system concepts. In order to be able to achieve the desired cleaning results within the shortest possible period of time, the effectiveness of the cleaning medium is usually enhanced by means of various physical processes that demonstrate effects of varying magnitude, such as spraying, ultrasound and flushing under pressure.
For manufacturing of implants, for example, it is advisable to use an intermediate cleaning step after each chip-removing machining process. This reduces the risk of any accumulation of deposits in the micron range on the parts, which may lead to tolerance deviations during further processing. Beyond this, intermediate cleaning prevents any mixing of lubricants and processing oils on the parts, which often causes cleaning problems. The quality of downstream mechanical processing is improved by intermediate parts cleaning. Final cleaning is usually executed by means of a validated process with an aqueous medium, which assures biocompatibility.
Economic Batch Processes
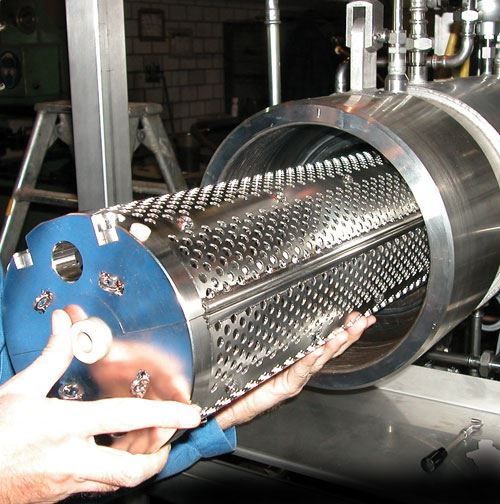
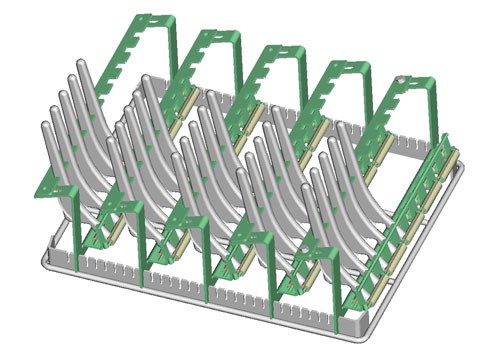
Batch processes are often used to clean medical devices in bulk goods baskets or in workpiece carriers that have been laid out specifically for the parts to be cleaned. In addition to the utilized process technology, the selected chemical and the duration of treatment, the cleaning rack also has a decisive effect on cleaning results and operating costs. It frequently offers potential for optimizing the cleaning process with regard to cleaning results, processing time and costs.
Cleaning baskets made of round wire are ideal for assuring quick and reliable removal of contamination. They allow for easy, uniform access to the workpieces by the cleaning agent so that the mechanical washing process can develop its full effectiveness and wash out film-like contamination and particulates as efficiently as possible. As opposed to closed containers or baskets made of perforated sheet metal, cleaning baskets made of round wire are also distinguished by significantly better draining characteristics. Consequently, considerably less contamination and cleaning agent is carried over, resulting in a longer service life for the cleaning bath, and thus improved cleaning system availability and efficiency.
Cleaning with Supercritical Carbon Dioxide
When parts are cleaned with supercritical carbon dioxide, the CO2 is used in an aggregation state in which its physical characteristics lie between the liquid and the gaseous state. While in this state, it demonstrates only minimal viscosity and surface tension. Both of these attributes are important prerequisites for mass transport, which makes it possible to remove contamination such as oils and greases from even the smallest cracks and pores.
Therefore, cleaning with supercritical carbon dioxide, which usually takes place within a temperature range of 20–40° C (68–104°F), is capable of achieving good results even with porous structures. Additionally, “biological” parameters are favorably influenced as a result of lower cytotoxicity values than those found in conventional cleaning processes. Uses for CO2 cleaning in medical engineering include, for example, intermediate and final cleaning of implants, instruments and components made of various materials such as metals and plastics. Amongst other things, a GMP validation package is part of the scope of delivery for manufacturers of systems for cleaning with supercritical CO2.
CO2 Snow—Dry and Residue-Free
Liquid carbon dioxide is used as a blasting medium for CO2 snow jet cleaning. It is expanded through a nozzle and accelerated to ultrasonic speeds with compressed air. Thanks to a combination of mechanical, thermal and chemical effects, the CO2 snow jet gently removes numerous types of film-like contamination and particulates when it strikes the surface in a dry and residue-free fashion. Its characteristics make CO2 snow jet technology suitable for cleaning and activating almost all materials, including metals, plastics, glass and ceramic substrates, and even finely structured surfaces. The jet stream can be well focused, and the process is, therefore, also capable of treating specific functional areas. An example of this focused treatment area can be seen when sealing and bonding surfaces without subjecting the entire component to the complex processing that is necessary to achieve the levels of cleanliness that are only required for the functional surfaces. The process’s good inline capabilities and minimal space requirements allow for easy integration of cleaning into the manufacturing process, thus ruling out the possibility or renewed contamination of the component, for example during transport or storage.
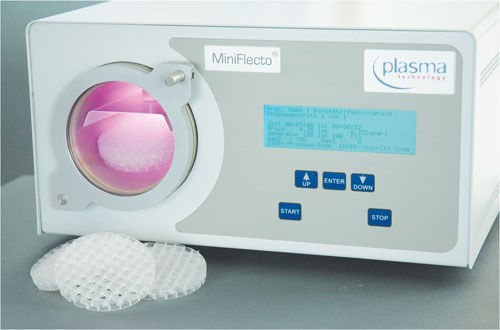
Plasma—More than Just Clean
Plasma is a gaseous mixture of atoms, molecules, ions and free electrons. Medical devices made of various materials, such as steel, non-ferrous metals, plastics, glass and ceramics, can be treated either in batch processes or as individual parts with this technology. Depending upon the application, various plasma gases can be used, by means of which the surface is simultaneously cleaned and activated. This dual function is based on both physical and chemical reactions involved in the process: The atoms released in the plasma “bombard” the surface of the component to be cleaned. This functions like a miniature sand-jet in the nano-range, thus removing organic contamination that adheres to the surface, such as oil and grease. At the same time, free ions and electrons are deposited on, and enter into a chemical bond with the surface. As a result, surface tension is adjusted to an ideal value for subsequent bonding, coating or printing processes.
Applications for biocompatible plasma in the field of medical engineering include final cleaning of stents, surgical and dental implants, as well as guide wires prior to coating with hydrogel or PTFE, increasing the surface energy of microtiter plates and other diagnostic instruments, silicone breast implants, catheters and syringes.
Read Next
5 Rules of Thumb for Buying CNC Machine Tools
Use these tips to carefully plan your machine tool purchases and to avoid regretting your decision later.
Read MoreSetting Up the Building Blocks for a Digital Factory
Woodward Inc. spent over a year developing an API to connect machines to its digital factory. Caron Engineering’s MiConnect has cut most of this process while also granting the shop greater access to machine information.
Read MoreBuilding Out a Foundation for Student Machinists
Autodesk and Haas have teamed up to produce an introductory course for students that covers the basics of CAD, CAM and CNC while providing them with a portfolio part.
Read More




























.jpg;maxWidth=300;quality=90)





