Citric Acid Safeguards Stainless Steel
This rendering shows a large passivation line.
Share








ECi Software Solutions, Inc.
Featured Content
View More
.png;maxWidth=45)
DMG MORI - Cincinnati
Featured Content
View More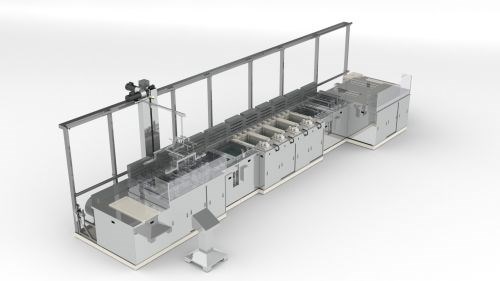
This rendering shows a large passivation line.
Citric acid is a substance that is growing in popularity for making stainless steel parts more corrosion-resistant, according to cleaning and finishing equipment maker Miraclean. An article from the company, “Passivation of Stainless Steel Parts: The Recipe for Success,” was just posted to MMS Online.
Passivation is a process that happens naturally. A chemically inert surface layer forms when stainless steel is exposed to an oxygen environment. The dedicated, industrial, shop-floor process of passivation simply involves forming this layer more effectively and more reliably.
Cleaning is crucial to this, the article's authors point out—which is why a passivation line typically gives cleaning careful attention. After cleaning, the actual passivation involves controlled contact between the stainless steel part and an acid.
Nitric acid has been the agent of choice. However, citric acid (found in citrus fruits, but not to be confused with vitamin C) has been gaining ground. Citric acid is safer and environmentally friendlier, the authors say, and it can also deliver shorter cycle times. Some shops that understand passivation well have both nitric and citric passivation tanks available.





.png;maxWidth=150)
























.jpg;maxWidth=300;quality=90)



